Powered by Citizen Science
Infectious Disease Diagnostic Development
How and why diagnostics are developed (and regulated)
Overview
Diagnostic tests play a critical role in identifying the cause of an infection, guiding treatment decisions, and preventing the spread of disease. But before a test makes it to a hospital, pharmacy, or home, it undergoes a long development journey — from research bench to regulatory approval to real-world implementation.
There are several regulatory and development pathways for infectious disease diagnostics, including In Vitro Diagnostics (IVDs), Laboratory Developed Tests (LDTs), and Research Use Only (RUO) tools. Each plays a different — but important — role in fighting infectious disease threats, improving microbiome health, and reducing antibiotic resistance.
Diagnostic Test Types
IVDs (In Vitro Diagnostics):
IVDs are subject to the highest level of regulatory scrutiny and must be reviewed and cleared, approved, or authorized by the FDA.
Key considerations include:
-
A clearly defined intended use, testing environment, and risk classificationExtensive analytical and clinical validation
-
Typically used in commercial or centralized lab settings
-
Because of their rigorous review, clinical laboratories can modify FDA-cleared IVDs under a minimal validation pathway to expand utility — such as adding additional specimen types or organisms to fit local patient populations.
LDTs (Laboratory Developed Tests):
LDTs are created within CLIA-certified laboratories and are not submitted to the FDA but still require thorough analytical and clinical performance evaluations. These tests are often used to meet urgent or niche diagnostic needs, especially when no commercial test exists.
RUO (Research Use Only):
RUO assays are designed strictly for experimental or research purposes, not for clinical decision-making. However, they can be an important starting point for assay development. A test may begin as RUO and, if successful, later undergo validation to become an LDT or an FDA-authorized IVD.
Key Phases of Diagnostic Development

Discovery & Feasibility: Researchers identify a biomarker (e.g., a bacterial gene or viral protein) that reliable signals infection.
Assay Design: Developers build and optimize a test to detect the biomarker reliably. At this stage, the intended use and ease of interpretation help determine the regulatory pathway the test must follow.
Analytical Validation: The test is evaluated under controlled and regulated laboratory conditions for sensitivity, specificity, accuracy, and reproducibility. This phase sets the assay’s performance standards — and locks in the design for all future use.
Clinical Validation: The test is trialed in real-world clinical settings include samples to ensure it works across all intended patient populations.
Regulatory Submission:
In the U.S., IVD developers may seek FDA clearance, approval, or Emergency Use Authorization (EUA). Internationally, CE-IVDR or country-specific regulations apply. Clinical laboratories may also create Laboratory Developed Tests (LDTs) or modify FDA-cleared assays to address unmet diagnostic needs. These fall under CLIA oversight and, in some states like New York, must meet additional public health standards through programs such as CLEP (Clinical Laboratory Evaluation Program).
⚠️ Note: Research Use Only (RUO) assays are not intended for patient care and are less regulated. They are not reviewed by FDA or state public health authorities. Look for disclaimers like “not evaluated by the FDA” — results from RUO tests should be interpreted with extreme caution and never used as the sole basis for treatment decisions.
Post-Market Surveillance/ Ongoing monitoring:
Even after diagnostic tests are in use, they are continuously monitored for performance, safety, and quality. The level of oversight depends on the testing environment — the simpler the setting (e.g., at-home tests), the more streamlined the monitoring. Complex lab-based tests typically undergo more rigorous ongoing review.
Why It Matters
Regulation ensures that diagnostic tests are accurate, safe, and trustworthy — protecting both patients and healthcare systems from misleading or harmful results. But regulation also adds time and cost, especially for diseases with small or emerging markets.
Just as important as accuracy is interpretability. A test isn’t useful unless the provider understands how to act on it, and the patient understands what it means. This is especially crucial for at-home and digital health tools, where clear instructions and responsible reporting are part of the diagnostic’s effectiveness.
That’s why innovation, including user-centered design and community involvement, is so critical. When patients, providers, and developers work together, we can build diagnostics that are not only technically sound, but also usable, accessible, and trusted.

Regulation Depends on Where the Test Is Used
Understanding Diagnostic Test Categories
Not all diagnostic tests are created — or regulated — equally. The setting in which a test is developed and used determines how much oversight it receives and what it can be used for. This includes not just who runs the test, but also where it is used:
-
Laboratory-based testing (in CLIA-certified labs)
-
Point-of-Care (POC) testing (in clinics, ERs, or at the bedside)
-
Over-the-Counter (OTC) or at-home tests (used without a provider)
Here’s how the three major regulatory categories compare:
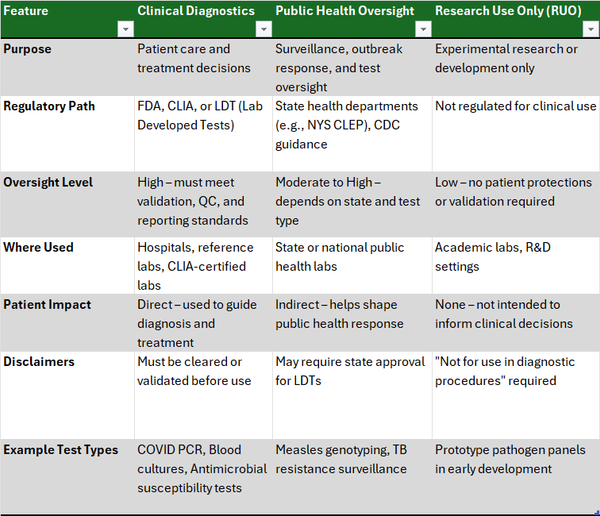
Clinical Diagnostics:
Used directly in patient care, these tests undergo rigorous validation and may be FDA-cleared, approved, or authorized depending on setting and risk.
-
Lab-based tests are often high-complexity and require CLIA certification.
-
POC (point of care) tests must be simple and CLIA-waived.
-
OTC (over the counter) tests must be designed for unsupervised use and include clear instructions.
Clinical labs may also create Laboratory Developed Tests (LDTs) under CLIA to meet specific diagnostic gaps. These require strong internal validation.
Example: Blood cultures, flu swabs, COVID-19 rapid tests.
Public Health Oversight
These diagnostics are reviewed or developed by state and federal public health agencies (e.g., NYS CLEP, CDC). They are often used for surveillance, outbreak control, or performance verification — not necessarily for individual clinical decisions.
Example: TB resistance panels, public health lab-based serologies.
Research Use Only (RUO)
RUO tests are for research and development only, not for patient care. They are not FDA-reviewed or CLIA-regulated, and must include disclaimers like “not for diagnostic use.” However, RUOs can be stepping stones to validated LDTs or FDA-authorized IVDs.
Example: Experimental multiplex PCR panels in development.

What Developers should know
Citizen Science Considerations

